| ОписΔFosB.svg |
English: The finished, wikilink annotated version is currently located at en:Template:Psychostimulant addiction; a more detailed image caption is located there as well.For external (off-wiki) use, a screenshot of the annotated version is located at File:Annotated ΔFosB.svg screenshot.png.
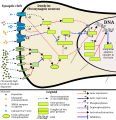
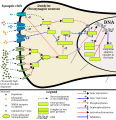
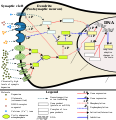
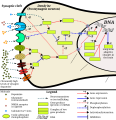
This image shows the signal transduction that occurs in nucleus accumbens D1-type medium spiny neurons that gives rise to psychostimulant addiction. This signaling cascade occurs during chronic high-dose use of certain dopaminergic stimulants (e.g., phenethylamine, methamphetamine, amphetamine, etc.).
Cocaine induces a slightly different signaling cascade than the one shown here.
Image references
- For comparison: KEGG Pathway – human cocaine addiction
- This 2015 review article on methamphetamine covers an analogous signal transduction pathway in the NAcc, as illustrated in figure 1.
- The effect of DA receptors 3,4, and 5 on this cascade is referenced in the following review article.[2]
- The phosphorylation of CREB through the CAMK branch is referenced in figure 2 from the following review article.[3]
- The use of JunD (instead of Jun, which KEGG uses) in the annotated template is based upon figure 4 from the following review article.[4]
- Cited publications
- ↑ Amphetamine addiction. Kyoto Encyclopedia of Genes and Genomes (KEGG). Kanehisa Laboratories (28 October 2019). Retrieved on 12 February 2020. "Amphetamine is a psychostimulant drug that exerts persistent addictive effects. Most addictive drugs increase extracellular concentrations of dopamine (DA) in nucleus accumbens (NAc) and medial prefrontal cortex (mPFC), projection areas of mesocorticolimbic DA neurons and key components of the "brain reward circuit". Amphetamine achieves this elevation in extracellular levels of DA by promoting efflux from synaptic terminals. Acute administration of amphetamine induces phosphorylation of cAMP response element-binding protein (CREB) and expression of a number of immediate early genes (IEGs), such as c-fos. The IEGs is [sic] likely to initiate downstream molecular events, which may have important roles in the induction and maintenance of addictive states. Chronic exposure to amphetamine induces a unique transcription factor delta FosB, which plays an essential role in long-term adaptive changes in the brain.
KEGG Pathway – human amphetamine addiction diagram"
- ↑ (May 2009). "Molecular mechanisms of psychostimulant-induced structural plasticity". Pharmacopsychiatry 42 Suppl 1: S69–S78. DOI:10.1055/s-0029-1202847. PMID 19434558. PMC: 2734446. "Figure 2: Molecular pathways implicated in the structural changes that occur as a result of exposure to drugs of abuse. Transcription factors, such as NFκB, ΔFosB, CREB, and MEF2 play a role in regulating changes in dendritic spines, and can be activated by a variety of signaling pathways. Neurotrophins can signal via receptor tyrosine kinases to activate the PI3K-Akt and Ras-ERK pathways, which ultimately regulate transcriptional activity, and possibly control actin cytoskeletal dynamics through regulation of the Rho family of small GTPases. Dopaminergic stimulation of D1 and D2 receptors can in turn act on some of these same pathways by activation of PKA and PKC. Structural plasticity induced by psychostimulants can therefore result from manipulation of several signaling pathways that impinge on actin assembly processes as well as gene expression. ...
Consistent with the involvement of dopamine in these cellular and behavioral processes associated with addiction, it is not surprising that induction of dendritic spines after repeated treatment with psychostimulants occurs in both D1- and D2-expressing MSNs (medium spiny neurons) [49]. Interestingly, however, the long-term stability of new spines appears to be greater in D1 + compared to D2 + neurons. The persistence of increased dendritic spines in D1-containing MSNs correlates with the stable induction of ΔFosB [61]. through the D1/DARPP-32/PP1 signaling pathway [51, 116]. A member of the Fos family of genes, ΔFosB is a highly stable transcription factor that accumulates following drug administration in brains areas associated with rewarding aspects of drugs of abuse, including the NAc [40, 69]. Overexpression of ΔFosB increases the rewarding properties of several classes of drugs of abuse, including psychostimulants, while blockade of ΔFosB causes a reduction in drug reward [66, 68]."
- ↑ (September 2009). "Chromatin regulation in drug addiction and depression". Dialogues in Clinical Neuroscience 11 (3): 257–268. PMID 19877494. PMC: 2834246. "Figure 2: Regulation of chromatin structure by drugs of abuse. Drug-induced signaling events are depicted for psychostimulants such as cocaine and amphetamine. These drugs increase cAMP levels in striatum, which activates protein kinase A (PKA) and leads to phosphorylation of its targets. This includes the cAMP response element binding protein (CREB), the phosphorylation of which induces its association with the histone acetyltransferase, CREB binding protein (CBP) to acetylate histones and facilitate gene activation. This is known to occur on many genes including fosB and c-fos in response to psychostimulant exposure. [ΔFosB] is also upregulated by chronic psychostimulant treatments, and is known to activate certain genes (eg, cdk5) and repress others (eg, c-fos) where it recruits HDAC1 as a corepressor. This repression of c-fos also involves increased repressive histone methylation, which is thought to occur via the induction of specific histone methyltransferases (HMTs). In addition, cocaine regulates the HMT, KMT1 C/G9a, which alters histone H3 methylation on K9. It is not yet known how cocaine regulates histone demethylases (HDM) or DNA methyltransferases (DNMTs). Cocaine also activates the mitogen activated protein kinase (MAPK) cascade, which through MSK1 can phosphorylate CREB and histone H3 at serine 10. Cocaine promotes H3 phosphorylation via a distinct pathway, whereby PKA activates protein phosphatase 2A, leading to the dephosphorylation of serine 97 of DARPP32. This causes DARPP32 to accumulate in the nucleus and inhibit protein phosphatase-1 (PP1) which normally dephosphorylates H3. Chronic exposure to psychostimulants increases glutamatergic stignaling from the prefrontal cortex to the NAc. Glutamatergic signaling elevates Ca2+ levels in NAc postsynaptic elements where it activates CaMK (calcium/calmodulin protein kinases) signaling, which, in addition to phosphorylating CREB, also phosphorylates HDAC5. This results in nuclear export of HDAC5 and increased histone acetylation on its target genes (eg, NK1R [NK1 or substance P receptor])."
- ↑ (October 2011). "Transcriptional and epigenetic mechanisms of addiction". Nature Reviews. Neuroscience 12 (11): 623–637. DOI:10.1038/nrn3111. PMID 21989194. PMC: 3272277. "Figure 4: Epigenetic basis of drug regulation of gene expression. The figure is based on the mechanisms by which chronic cocaine, through ΔFosB, activates the cdk5 gene (top) and represses the c-fos gene (bottom). Top: ΔFosB binds to the cdk5 gene and recruits several co-activators, including CBP (CREB binding protein) — a type of histone acetyltransferase (HAT) leading to increased histone acetylation, transcription factor BRG1 (also known as brahma-related gene 1) — a type of chromatin remodeling factor — and SUG1 (proteasome 26S ATPase subunit 5), another type of chromatin regulatory protein. ΔFosB also represses G9a expression, leading to reduced repressive histone methylation at the cdk5 gene. The net result is gene activation and increased CDK5 expression. Bottom: In contrast, ΔFosB binds to the c-fos gene and recruits several co-repressors, including HDAC1 (histone deacetylase 1) and SIRT 1 (sirtuin 1). The gene also shows increased G9a binding and repressive histone methylation (despite global decreases in these marks). The net result is c-fos gene repression. As transcriptional regulatory complexes contain dozens or hundreds of proteins, much further work is needed to further define the activational and repressive complexes that cocaine recruits to particular genes to mediate their transcriptional regulation and to explore the range of distinct activational and repressive complexes involved in cocaine action."
|